Moderna says will request US, Europe vaccine authorization Monday
समयपोष्ट
२०७७ मंसिर १५ गते २१:२०
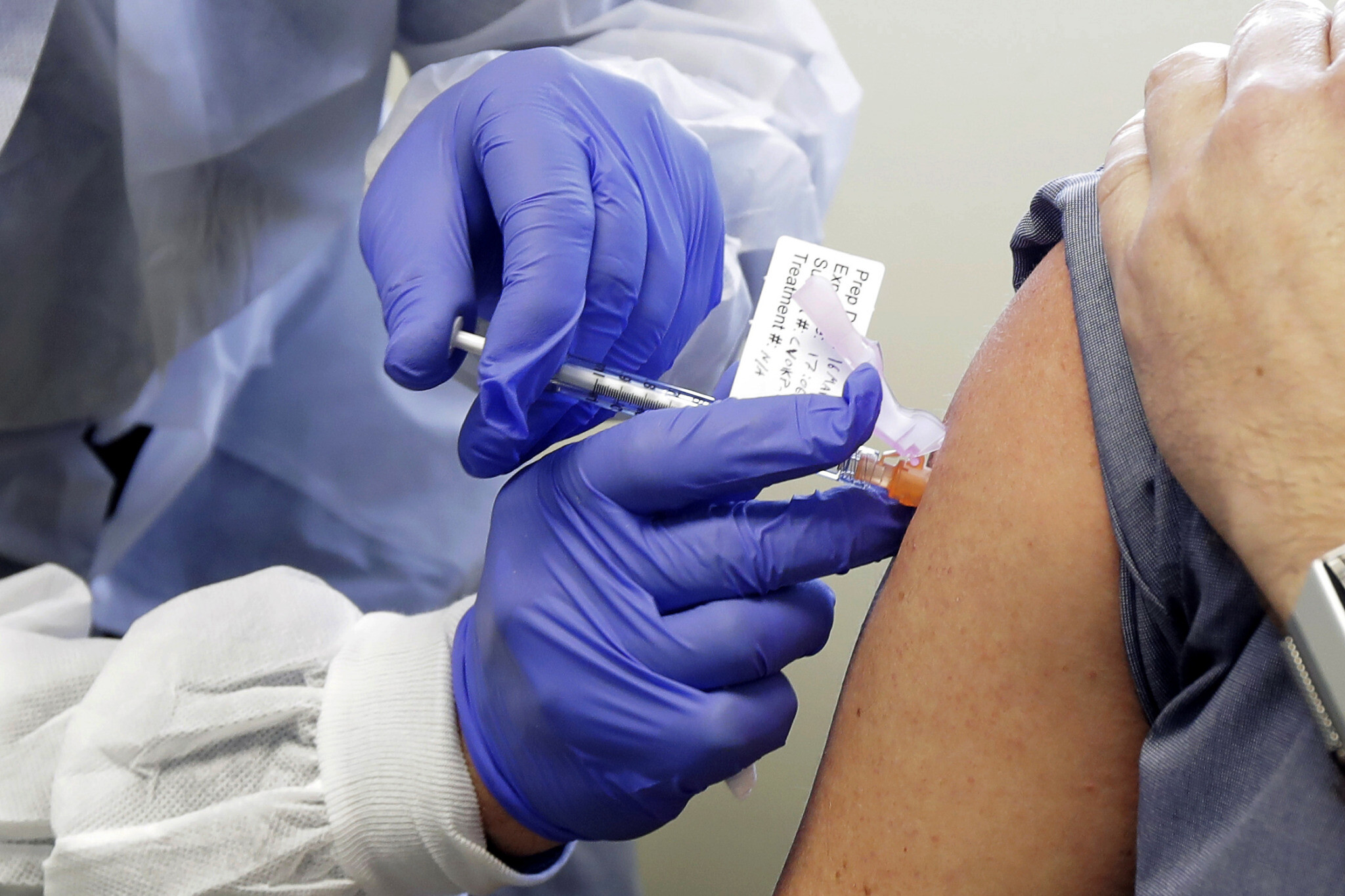
US firm Moderna said it would file requests for authorization of its Covid-19 vaccine in the United States and Europe on Monday, after full results confirmed a high efficacy estimated at 94.1 percent.
“Moderna plans today to request EUA (Emergency Use Authorization) from the US FDA (Food and Drug Administration),” Moderna said in a statement, adding it would also “apply for a conditional marketing authorization with the European Medicines Agency (EMA).”






























प्रतिक्रिया